-
Change Control Note (CCN)
-
Deviations
-
CAPA
QualZenTM
-
Lab Incidents
-
OOS/OOT
-
Market Complaints
21 CFR Part 11 compliant
Facilitates cross functional teams assessment
Dynamic Report Generation
Risk Mitigation
Accelerate QualZenTM with other ZenVectorTM Products
We have built our modules in a way when used together they can communicate with each other seamlessly.
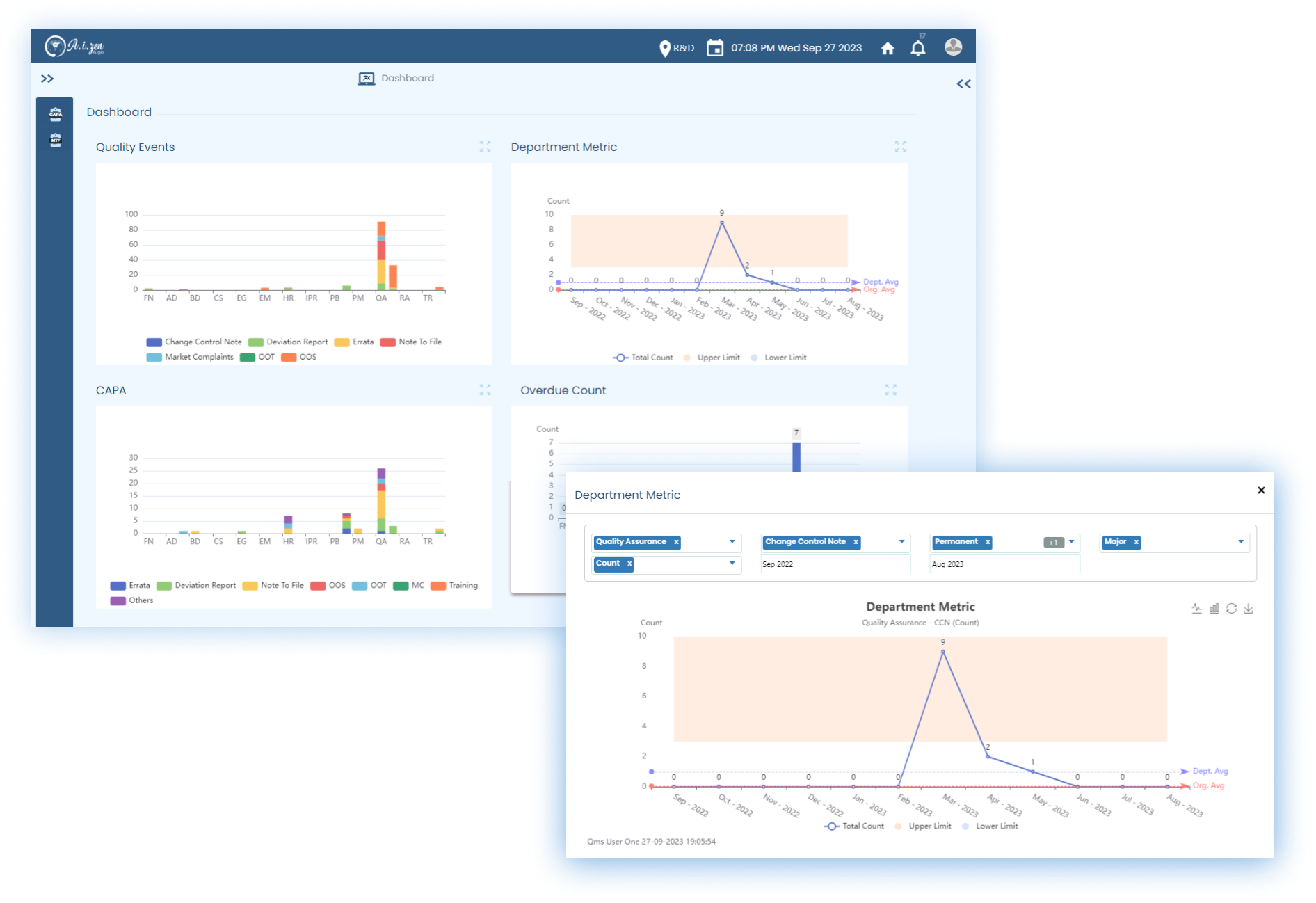
- QualZenTM integrates with DocuZenTM and EduZenTM to manage quality events seamlessly.
- If any change is implemented in SOP through a quality related event in QualZenTM, the same will directly get linked with the EduZenTM module within the ZenVectorTM platform, so that training can be provided to the workforce on the revised SOP.
- QualZenTM links with DocuZenTM to have all the quality events have the relevant SOP or document numbers mentioned within their records.
- Automatic initiation of transaction in QualZenTM upon OOS/OOT event trigger in LabZenTM.
Product Differentiators
Recommendations
NLP and AI/ML capabilities map the previous quality events to provide recommendations on assessing the Change Control, Incidents and related Quality Events.
Integrations
Integration with DocuZen™, EduZen™ and AuditZen™ applications.
Quality Event Management
Manages all quality related events, like: Change controls, Incidents, CAPAs, Market complaints, and OOS/OOT.
Workflow Management
Facilitates end-to-end quality event workflow management and ensures that complete audit-trail is maintained.
GMP Adherence
Eliminates inefficient manual systems and ensures adherence to the prevailing c-GMP.
21 CFR Part 11 Compliance
Ensures compliance with 21 CFR Part 11.
Flexible Report Generation
Reassignments & Extensions.
Impact Point Assessment
Integrated CAPA flow, Effective Checks.
Get Started With QualZenTM
We have built all the tools with unique Competitive feature you need to manage your important data


