-
Workflow Automation
-
Instrument Integration
-
Inventory management
LabZenTM
-
Efficient Sample Management
-
Data Security
-
Tracking and Exchanging
Laboratory data
21 CFR Part 11 Complaint
Quality Control and Regulatory Compliant
Scalable and Configurable
Effective Reporting System
Accelerate LabZenTM with other ZenVectorTM Products
We have built our modules in a way when used together they can communicate with each other seamlessly.
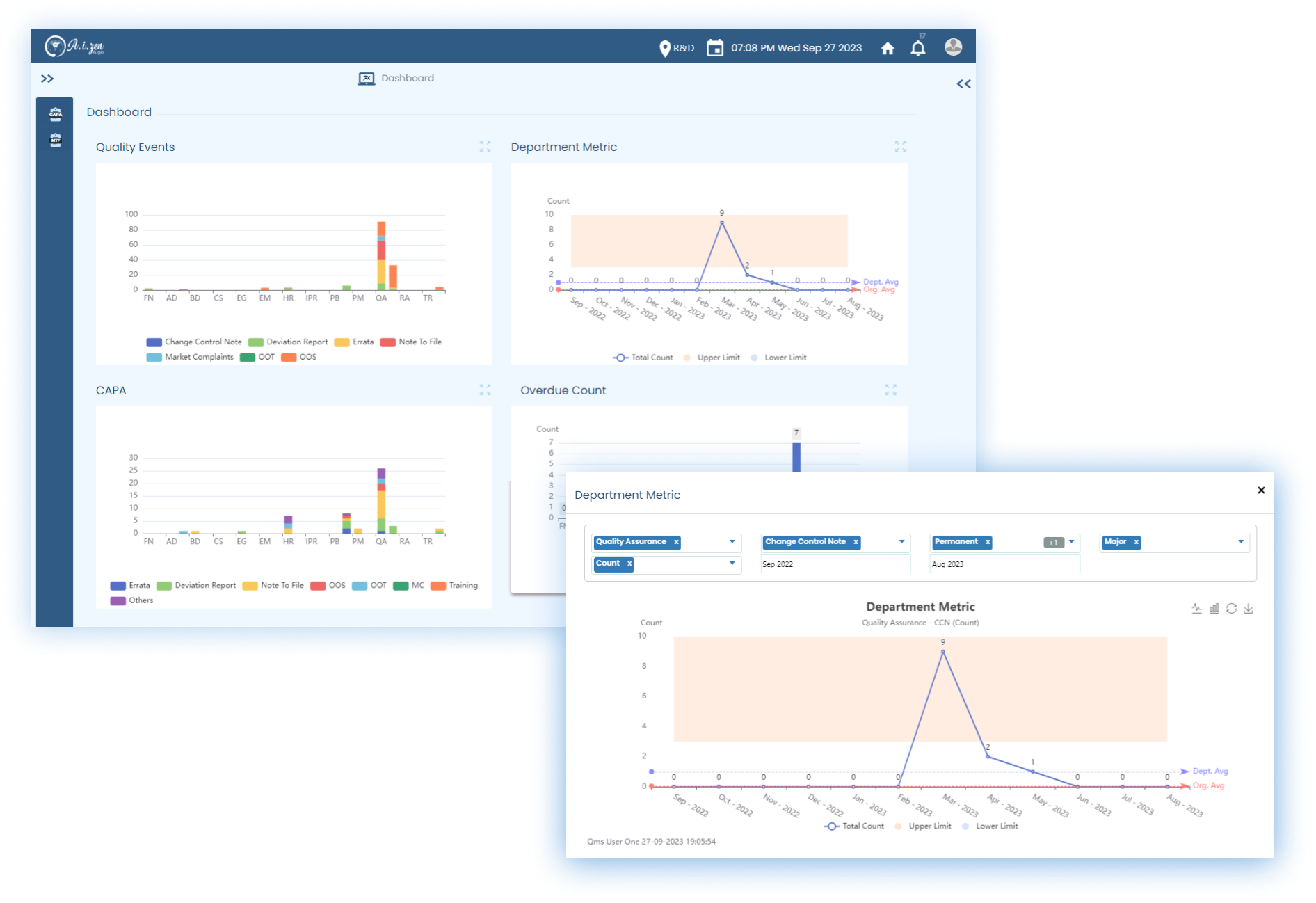
- AiZen Algo's LabZenTM can integrate with QualZenTM to assign and oversee CAPAs (Corrective & Preventive actions) assigned to various enterprise-wide stakeholders.
- Incidence of OOS and OOT and their investigation related metrics get reflected on the QualZenTM dashboards to provide an enterprise-wide quality and compliance view to QA teams.
- LIMS integrated with with DocuZenTM DMS to fetch documents related to Instrument SOPs, Protocols, Methods and other reference material essential to execute Quality Control and Stability related tests.
- EduZenTM provides Analyst qualification related metrics to LabZenTM to do fit-for-purpose task and work flow assignments to Lab Analysts.
- LabZenTM can be integrated with NoteZenTM for seamless text methods.
Product Differentiators
Samples Management
Bio Samples, Raw Materials, Chemicals, Finished Drugs,
Reference & Working Standards, Solutions, Stability etc.
Samples Management
Bio Samples, Raw Materials, Chemicals, Finished Drugs, Reference & Working Standards, Solutions, Stability etc.
Robust chain of custody
Roles and privileges based access, Complete trail of sample life cycle.
Automated Resource Management
Thorough, real-time inventory of all supplies, laboratory equipment etc.
Dynamic Configurability
Dynamic configuration of review and approval workflows.
Automated links to quality events
Investigation and closure on all types of Out of Specification (OOS) and Out of Trend (OOT) test results.
LIMS Triad
Lab Analysis Lifecyle, Resource Management, Investigative Analysis
Automated Equipment Interfaces
Measurement interfaces to CDS, SDMS, Particle Analysers, All lab apparatus & measuring devices.
Regulatory compliances
21 CFR Part 11, GLP Requirements
Get Started With LabZenTM
We have built all the tools with unique Competitive feature you need to manage your important data


